Organoid model systems are taking research to the next dimension
The term organoid first gained use in the mid-1960?s to describe cell aggregates observed during dissociation and re-aggregation experiments in classic developmental biology. Today, the term organoid refers to three-dimensional structures containing multiple organotypic cell types, recapitulating some of
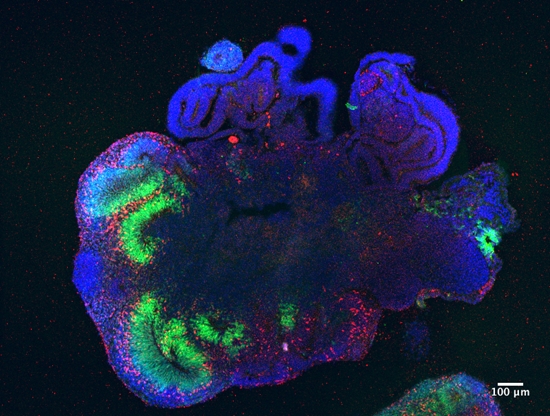
Using the novel technology of three-dimensional (3D) “mini-organs”, termed organoids, scientists are getting closer to understanding the detailed mechanistic pathways of how stem cells form and maintain complex tissues like organs.
During culture, organoids self-organize from stem cell populations maintaining the actively proliferating stem cells as well as differentiating into more mature cell types. Signaling gradients are established through the emergence of mature secretory cell types which allow continued renewal and proliferation of the stem cell population and maintenance of the stem cell niche.
Organoids can be formed from either adult stem cells or differentiated from pluripotent stem cells giving insights into how organs form during development as well as the homeostatic mechanisms of the adult tissues.
What are organoids?
The term organoid first gained use in the mid-1960’s to describe cell aggregates observed during dissociation and re-aggregation experiments in classic developmental biology.
Today, the term organoid refers to three-dimensional structures containing multiple organotypic cell types, recapitulating some of the function and structure of the in vivo organ.
Due to the preservation of key cell types and structures, organoids offer a highly physiologically relevant model system recreating the conditions observed in the organ. Additionally, organoids have been shown to be remarkably genetically stable over multiple passages retaining normal chromosome number and karyotype with a very low rate of mutation. To date, organoids have been grown from brain, retina, stomach, lung, liver, pancreas, small intestine, colon, and kidney among other tissues.
Why work with organoids?
As organoid culture techniques continue to be adopted and new tools for their manipulation are developed, organoids present themselves as a highly valuable technology complimentary to animal models and 2D culture.
Although a wide array of tools exists for the manipulation of traditional 2D cultures, they often lack the full complement of differentiated cell types observed in vivo and have limited potential for expansion and maintenance in vitro. The use of immortal cell lines avoids the limited replicative potential of in vitro culture, but tends to accumulate additional mutations over time and as such do not accurately reflect the true in vivo conditions.
Conversely, although animal models recreate the cellular complexity of the in vitro organ, tools for manipulation are often challenging, or not available, and the output data is often limited to endpoint observations or complicated by multiple organ systems. Additionally, animal models often do not reflect what is observed in humans resulting in extra experimentation and the unnecessary sacrifice of animals.
Organoids provide both increased cellular complexity, as well as tools for manipulation, giving a complementary system to existing techniques. Currently, tools exist for the transformation and transduction of organoid cultures through the use of retro- and lentiviral vectors allowing for the knock-in of reporter genes and conditional expression systems.
Additionally, precise genome editing can be achieved through the use of CRISPR/Cas9 technologies allowing for the introduction or correction of specific genetic changes. Co-infection systems have been developed for both bacteria and viruses allowing the study of infection models such as the use of cerebral organoids in the study of Zika virus infection.
Additionally, co-culture systems have been developed for the incorporation of other tissues including neurons and immune cells for several organoid models, mimicking the complex signaling contributions and interactions of the surrounding tissues.
The continuing development of high-throughput techniques presents the opportunity to use organoids as a highly physiologically relevant model for screening small molecule libraries during drug development.
Organoids have the potential to complement or reduce animal studies during early experimentation saving both resources and experimental time. The development of more functional assays is enabling organoids to be used in precision medicine to predict patient response to treatment. As new tools and culture conditions continue to be developed, organoids are increasingly being recognized as an invaluable tool to complement existing techniques.
Refernce:
1 – Biotechin Asia


ارسال به دوستان